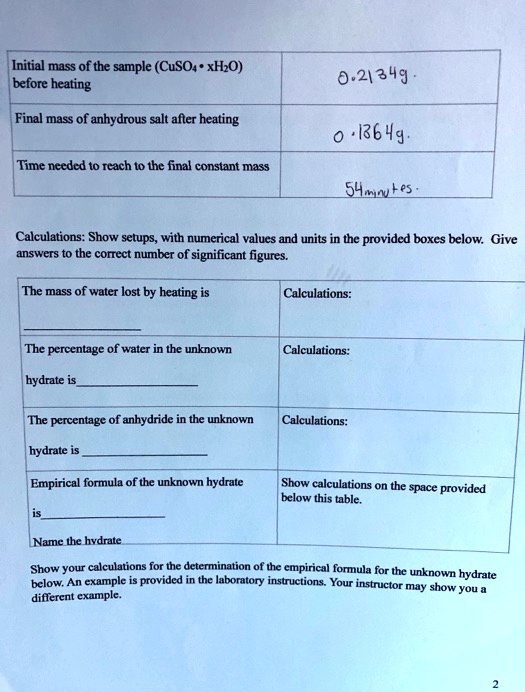
SOLVED: Initial mass of the sample (CuSO4 * xHO) before beating 0.21349 Final mass of anhydrous salt after heating Time needed t0 reach t0 the final constant mass S41uLes Calculations: Show setups;

A treatise on the theory of solution including the phenomena of electrolysis . °6 and pass into the anhydrous salt andwater. Another hydrate, Na2S04. 7 HgO can be obtained byadding alcohol to

One mole of anhydrous salt AB dissolves in water and librates 21.0 J mol^-1 of heat. The value of ΔHydration AB is - 29.4 J mol^-1 . The heat of dissolution of

thermodynamics - Why is it that the enthalpy of hydration equals the difference of solvation enthalpies of anhydrous and hydrous salts - Chemistry Stack Exchange

SOLVED: Formula (Molar) mass of anhydrous salt (on bottle) Number of grams of HzO per 100.0 g hydrate *49 14.8 Number of moles HzO per 100.0 g hydrate 1q.8 18.0 Da moles

One mole of anhydrous salt AB dissolves in water and librates 21.0 J mol ^-1 of heat. The value of Δ H(hydration) of AB is - 29.4 J mol ^-1 . The


.PNG)











:max_bytes(150000):strip_icc()/anhydrous-chemistry-definition-603387_final-46e5c09532c045568b0a07dfddba7397.png)
