Same Element Different Atom- Isotopes All atoms of a particular element are not exactly alike. Some elements have atoms with different masses (isotopes) - ppt download

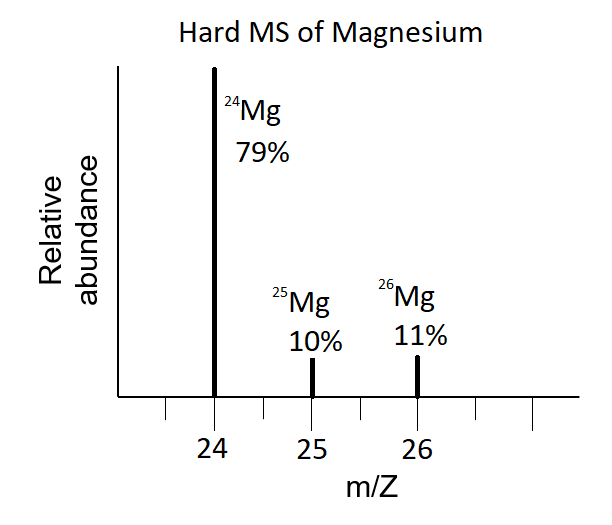
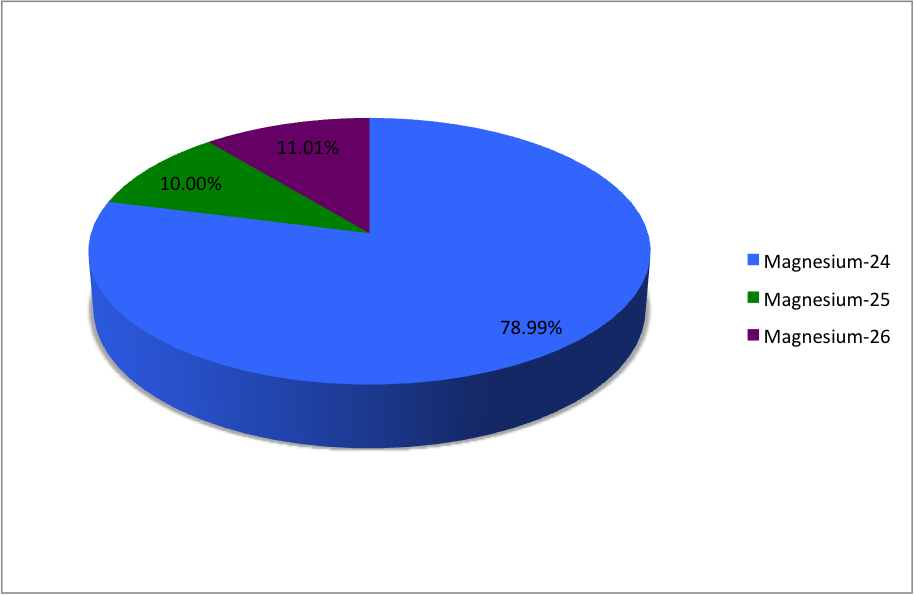
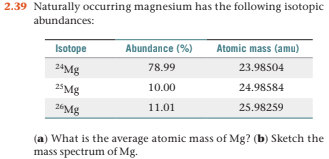
Magnesium has three naturally occurring isotopes with the following masses and natural abundances: \begin{array}{|c|c|c|} \hline \text{Isotope} & \text{Mass (amu)} & \text{Abundance (%)} \\ \hline \text{Mg-24} & \text{23.9850} & \text{78.99} \\ \hline \t
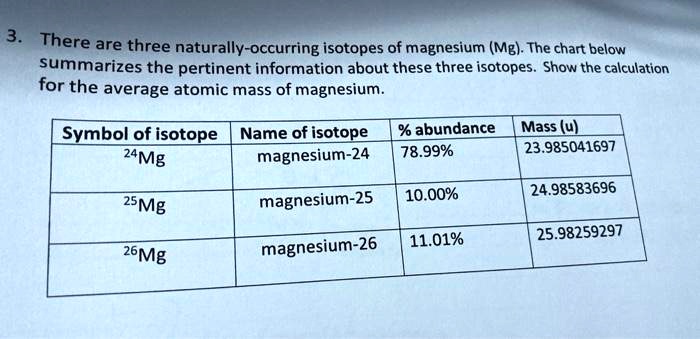
SOLVED: There are three naturally-occurring isotopes of magnesium (Mg) The chart below summarizes the pertinent information about these three isotopes Show the calculation for - the average atomic mass of magnesium. Symbol
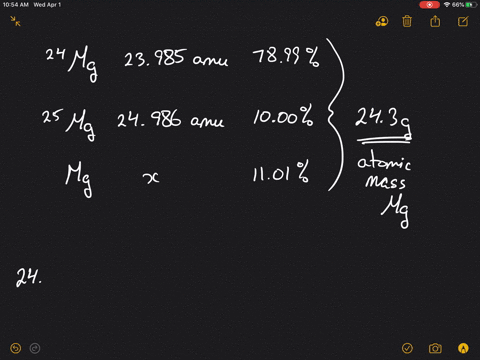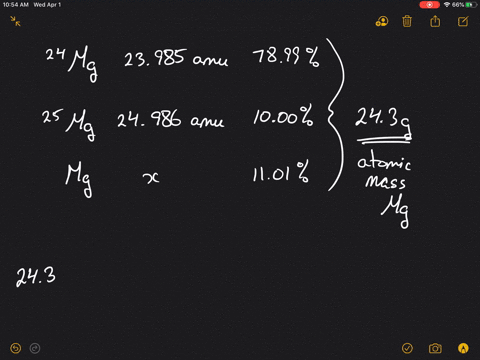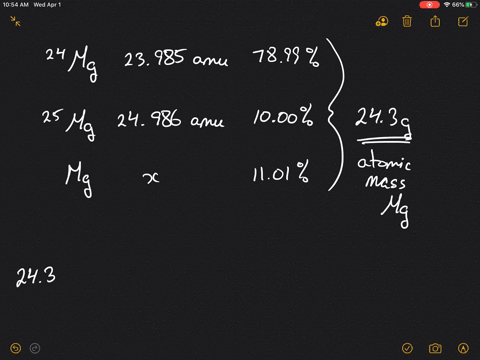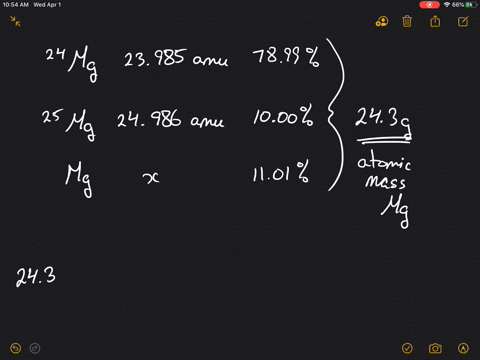
SOLVED:Magnesium has three naturally occurring isotopes: ^24 Mg (23.985 amu) with 78.99% abundance, ^{25} \mathrm{Mg} (24.986 amu) with 10.00% abundance, and a third with 11.01% abundance. Look up the atomic mass of
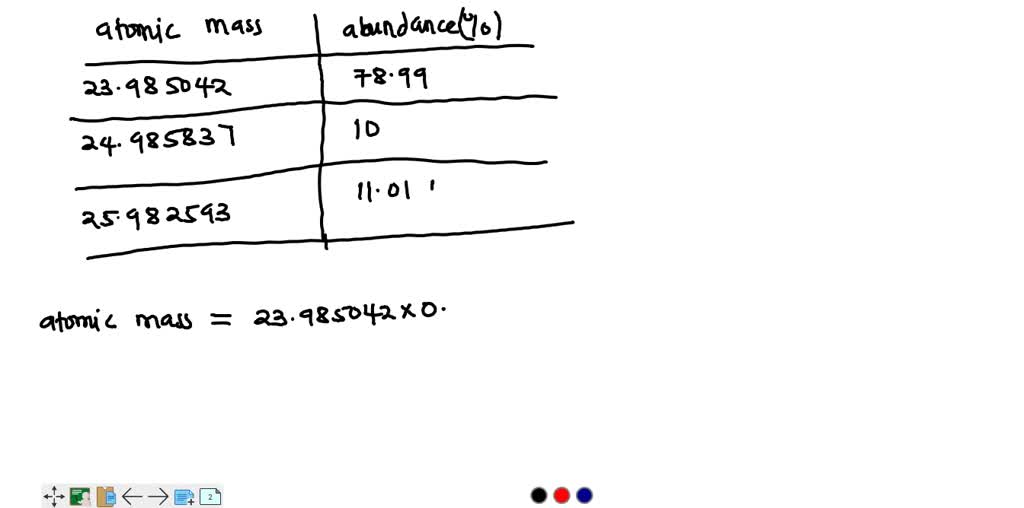
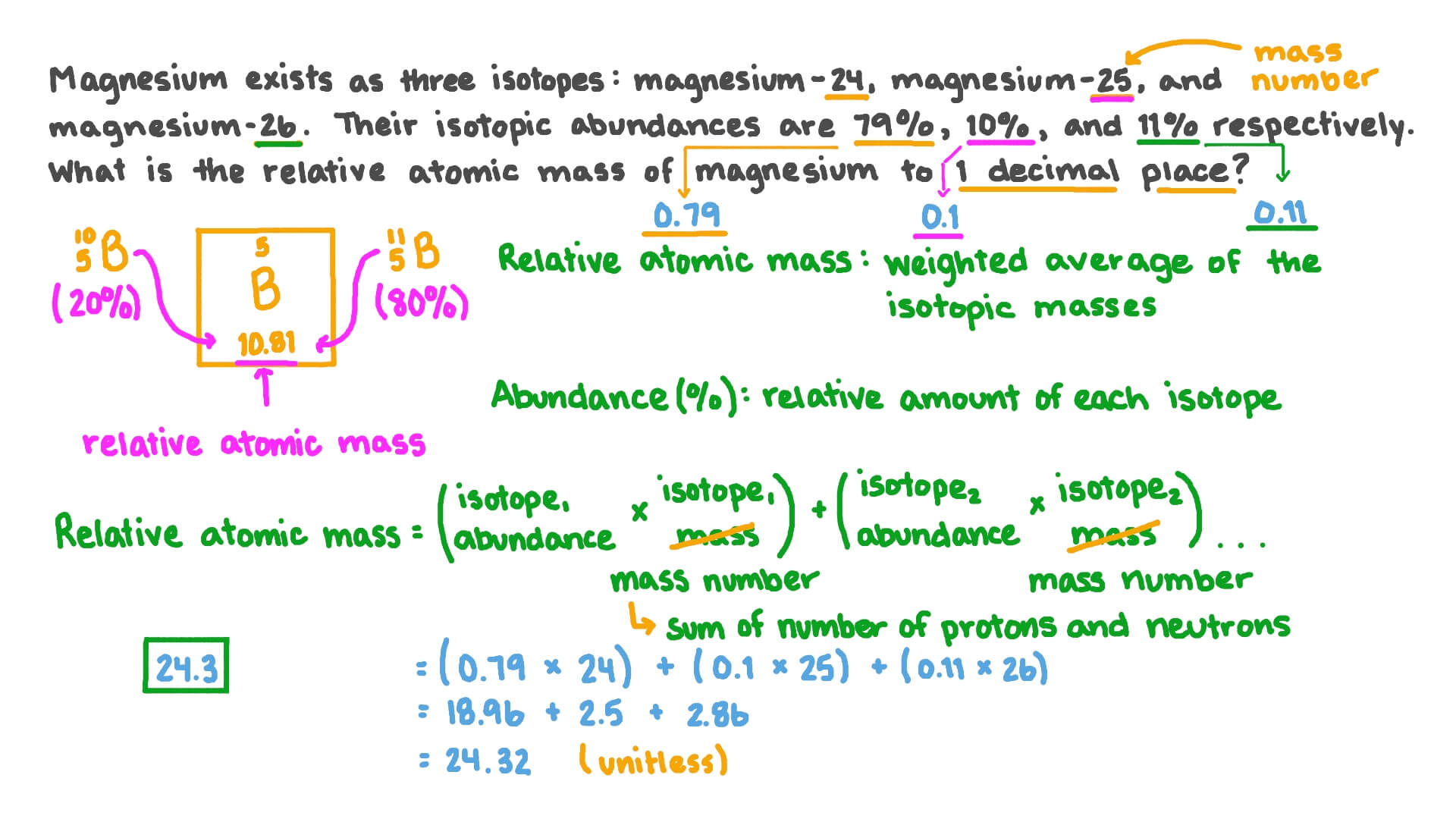
SOLVED: Magnesium has three natural isotopes. Their abundance percentages and masses in nature is as follows: 78.99%; 23.985042 akb, 10.00%; 24.985837 akb, 11.01%; 25.982593 akb. Accordingly, the weighted average atomic mass of magnesium calculate.

SOLVED: Naturally occurring magnesium has an atomic mass of 24.312 and consists of three isotopes. The major isotope is 24Mg, natural abundance 78.99%, relative atomic mass 23.98504. The next most abundant isotope

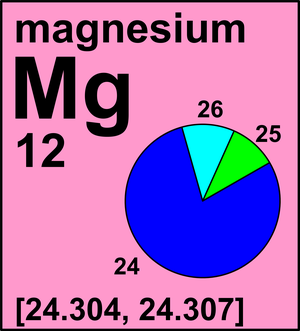
In a periodic table the average atomic mass of magnesium is given as 24.312 u. The average value is based on their relative natural abundance on earth. The three isotopes and their