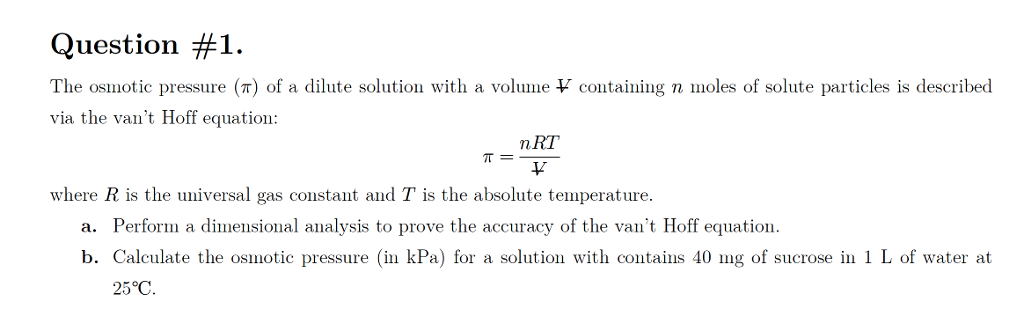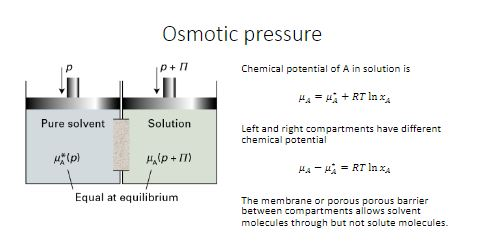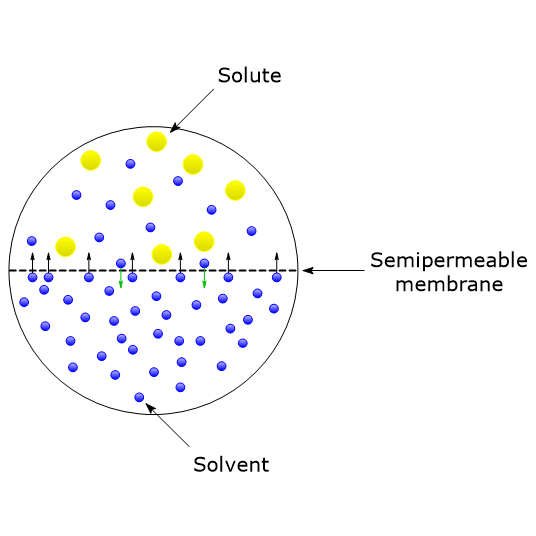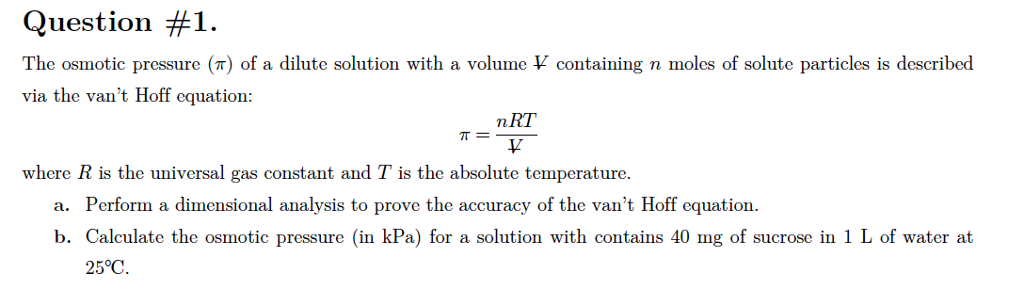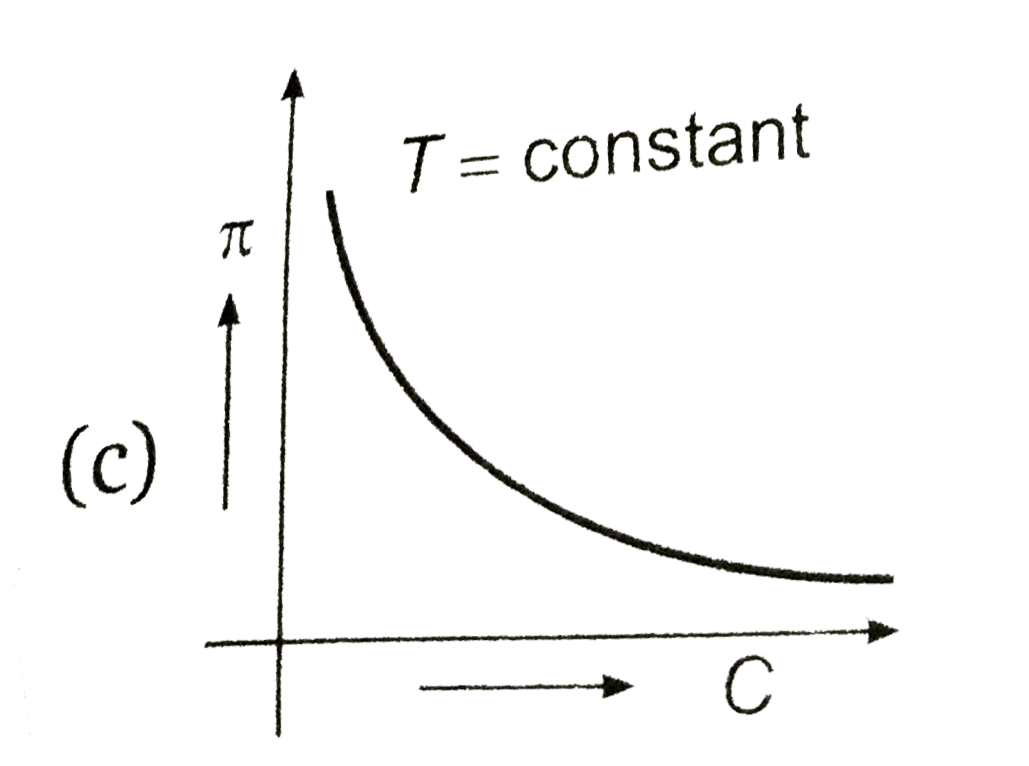
van't Hoff proved that osmotic pressure (pi) is a colligative property. For an ideal solution, osmotic pressure(pi) is helpful to determine that molecular mass of solute using M(B)=(W(B)RT)/(pi.V) Relation can exxpressed by

Vant Hoff formula - For calculation of Osmotic pressure ... ( Note: Maximum contribution of Plasma osmolarity is by sodi… in 2022 | Osmotic pressure, Gas constant, Pressure

Calculate the amount of CaCl2 (van't Hoff factor i = 2.47 ) dissolved in 2.5 L solution so that its osmotic pressure at 300K is 0.75 atmosphere.Given: Molar mass of CaCl2 is

PPT - Osmotic pressure – van't Hoff equation : = g C R T Where: - osmotic pressure (atm or mm Hg) PowerPoint Presentation - ID:4239208
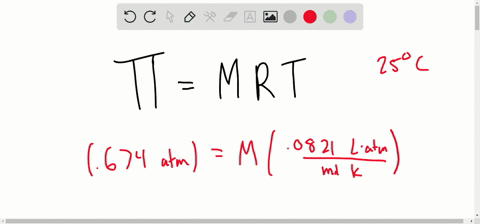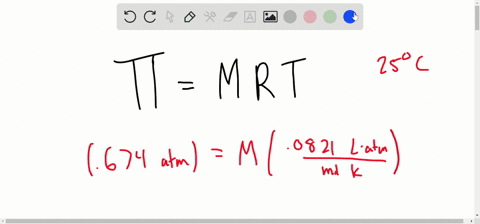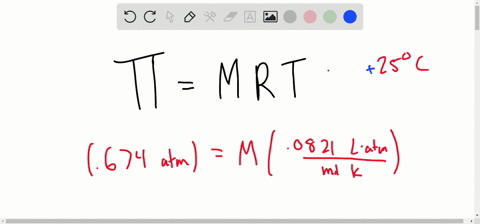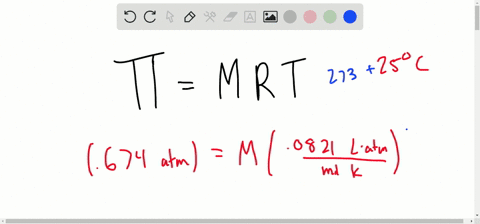
SOLVED:Determine the molarity of each of the following solutions from its osmotic pressure at 25^∘ C . Include the van 't Hoff factor for the solution when the factor is given. a.
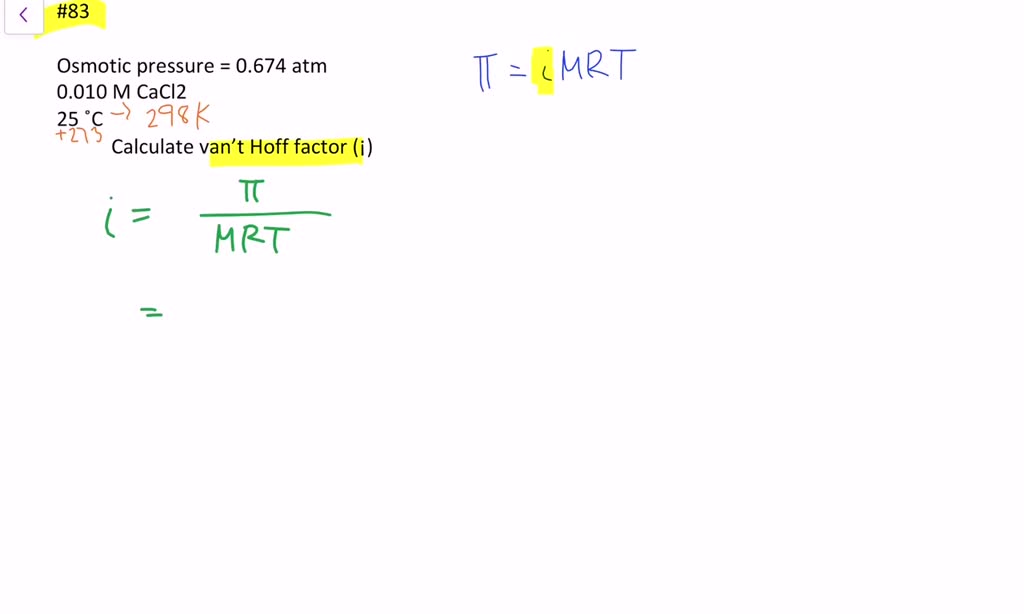
SOLVED:The osmotic pressure of a 0.010M aqueous solution of CaCl2 is found to be 0.674 atm at 25^∘ C . Calculate the van't Hoff factor, i, for the solution.
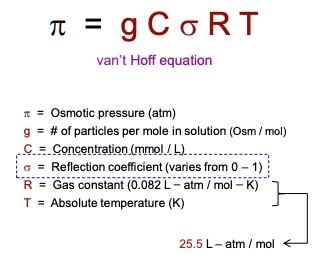
SOLVED: TC = g C o RT van't Hoff equation Osmotic pressure (atm) of particles per mole solution (Osm mol) Concentration(mmol Reflection coefficient (vares from Gas constant 082 atm mol Absolute temperature (