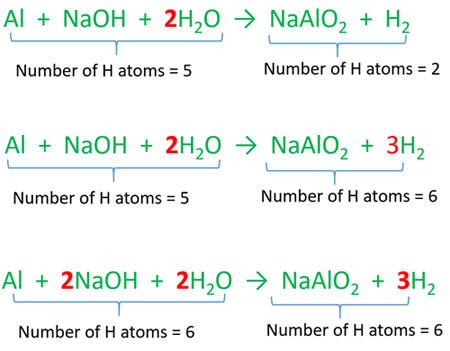
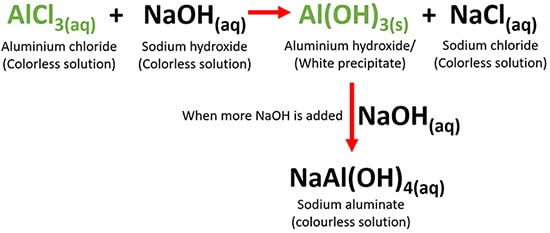
Name the gas in each of the following:The gas evolved on reaction of aluminium with boiling concentrated caustic alkali solution

The drain cleaner, Drainex contains small bits of aluminum which react with caustic soda to produce dihydrogen. What volume of dihydrogen at 20^o C and one bar will be released when 0.15
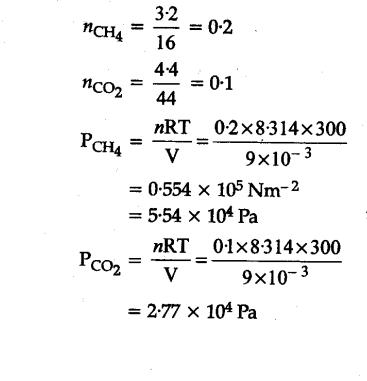
The drain cleaner Drainex contains small bits of aluminium which reacts with caustic soda to produce dihydrogen gas. What volume of dihydrogen at 20°C and 1 bar will be released when 0.15
Homemade Hydrogen | EXPERIMENT | Aluminium foil & Caustic soda | Crazy Reaction | KesPra ✓ - YouTube
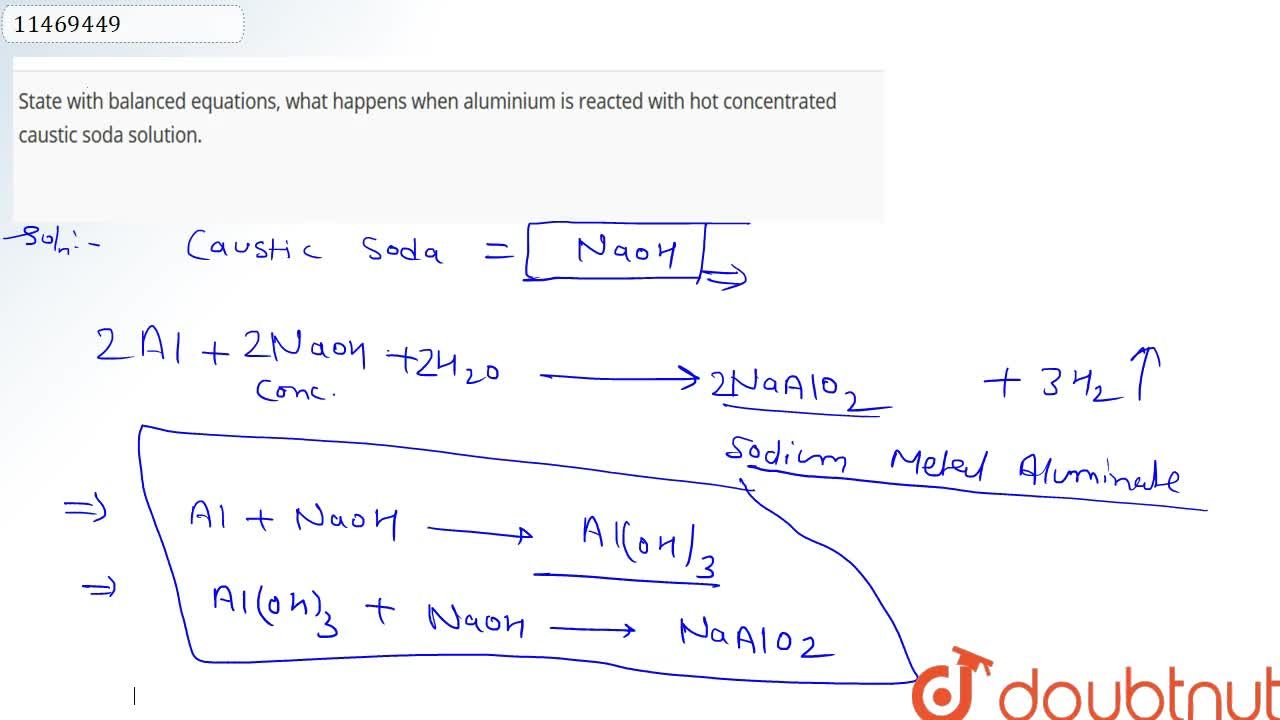
i) State with balanced equation, what happens when (a) Aluminium is reacted with hot conc caustic soda solution. - Sarthaks eConnect | Largest Online Education Community