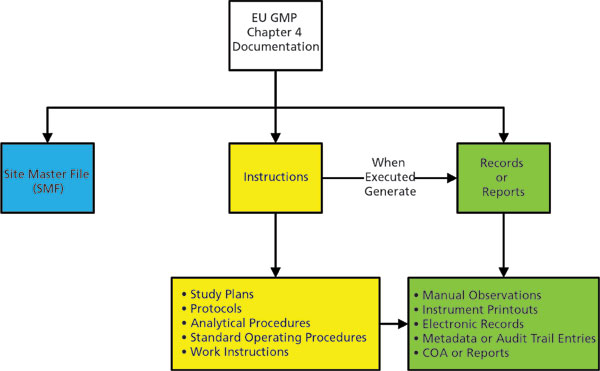
Data Integrity Awareness: Good Documentation Practices - LearnGxP: Accredited Online Life Science Training Courses
BioInsights - Assuring manufacturing and data integrity for cell and gene therapy regulatory compliance
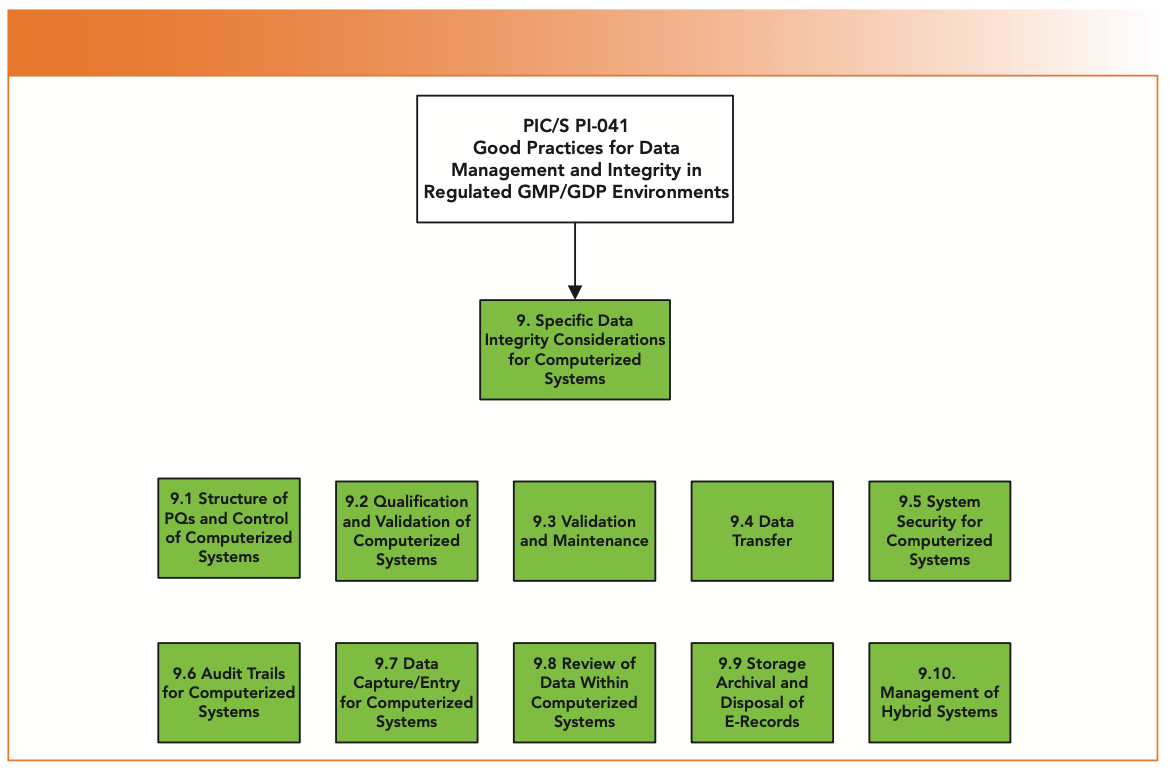
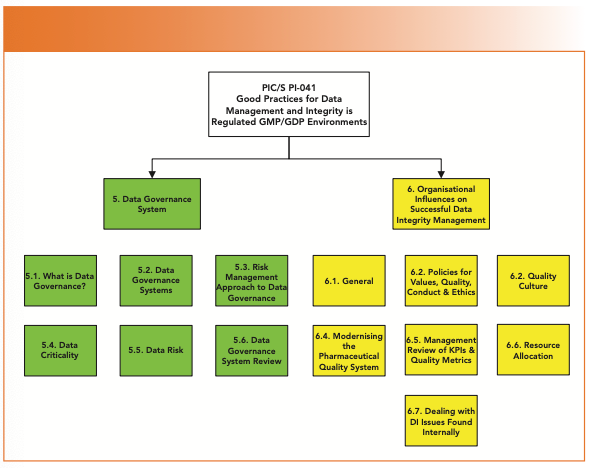
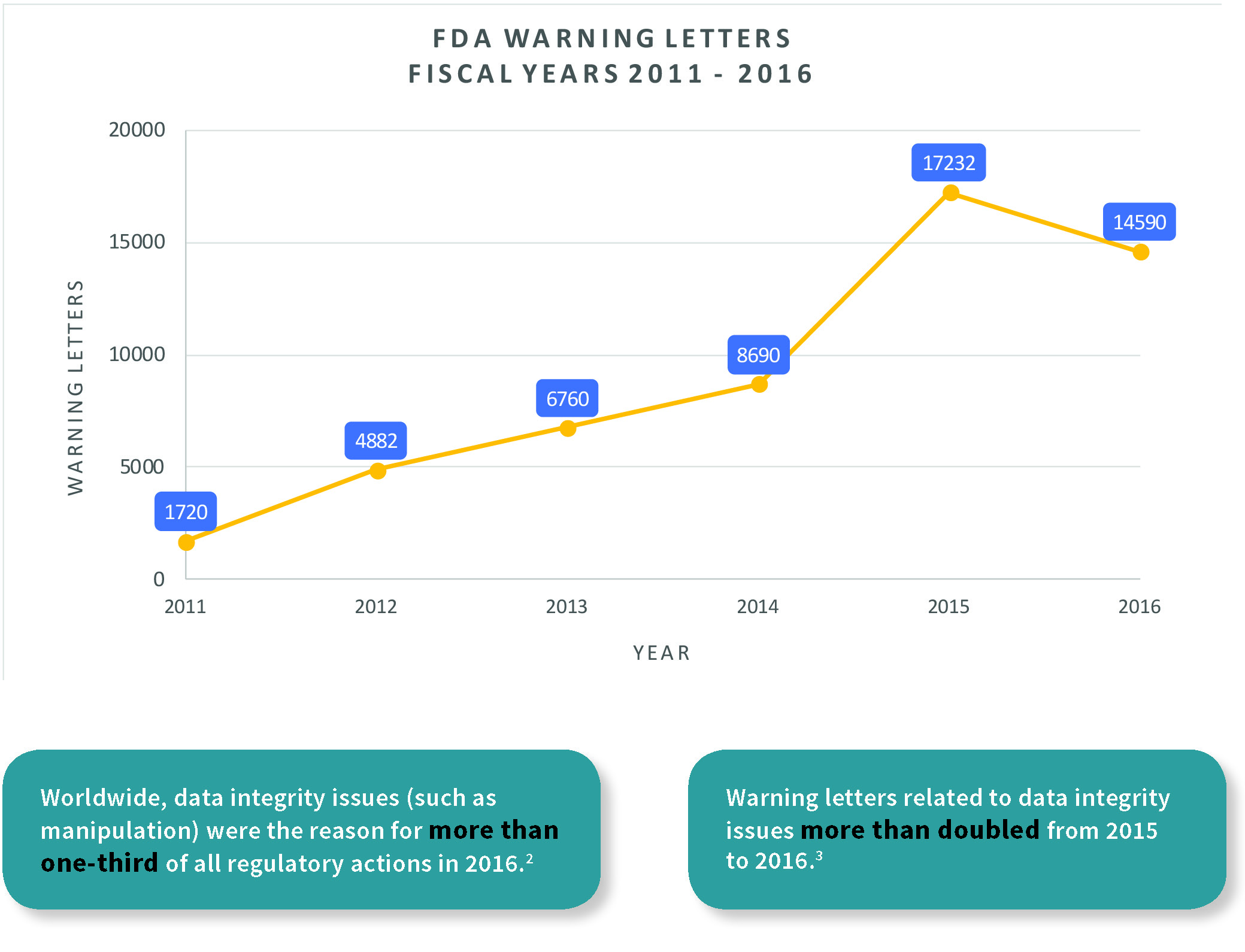
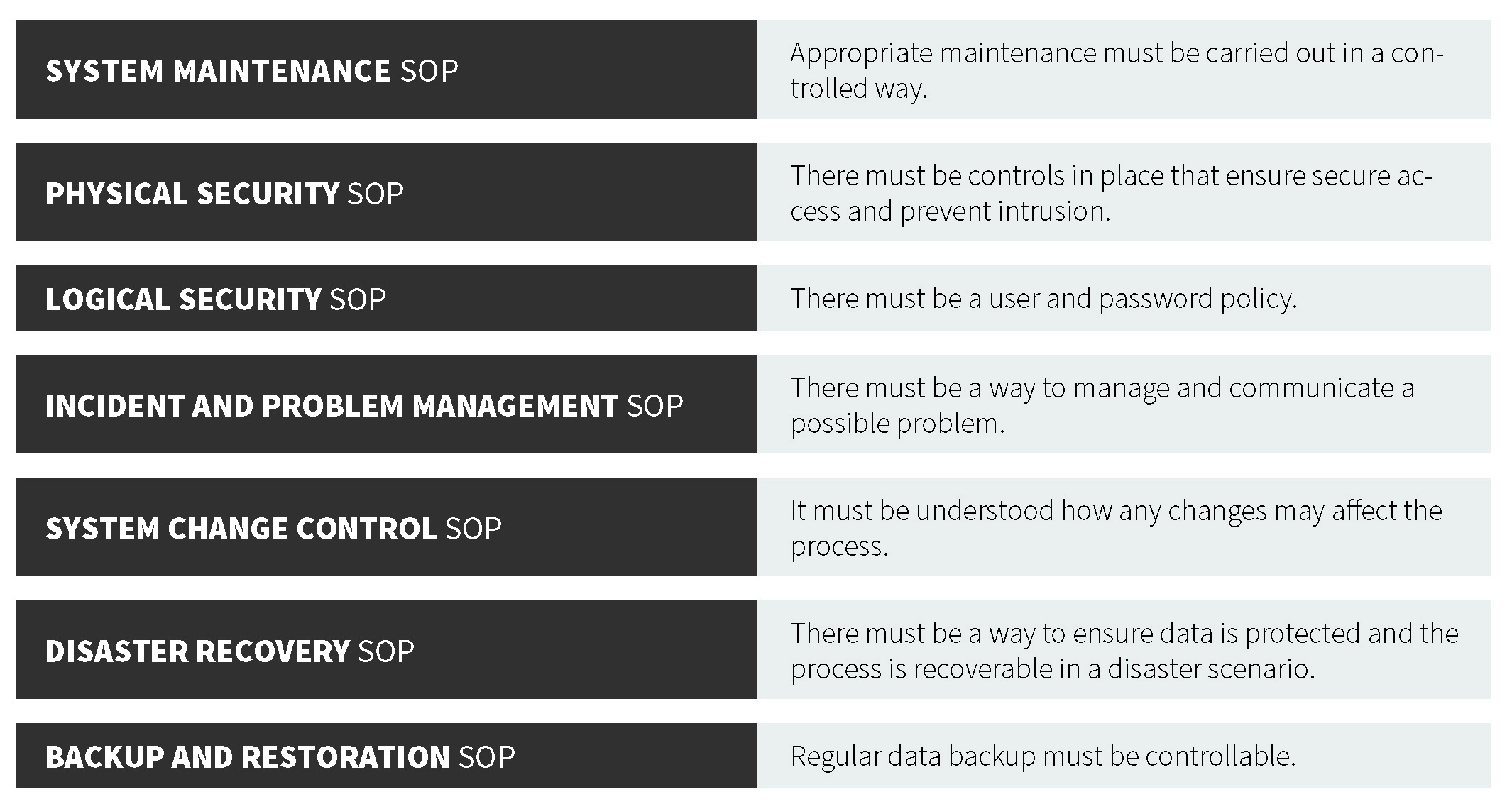
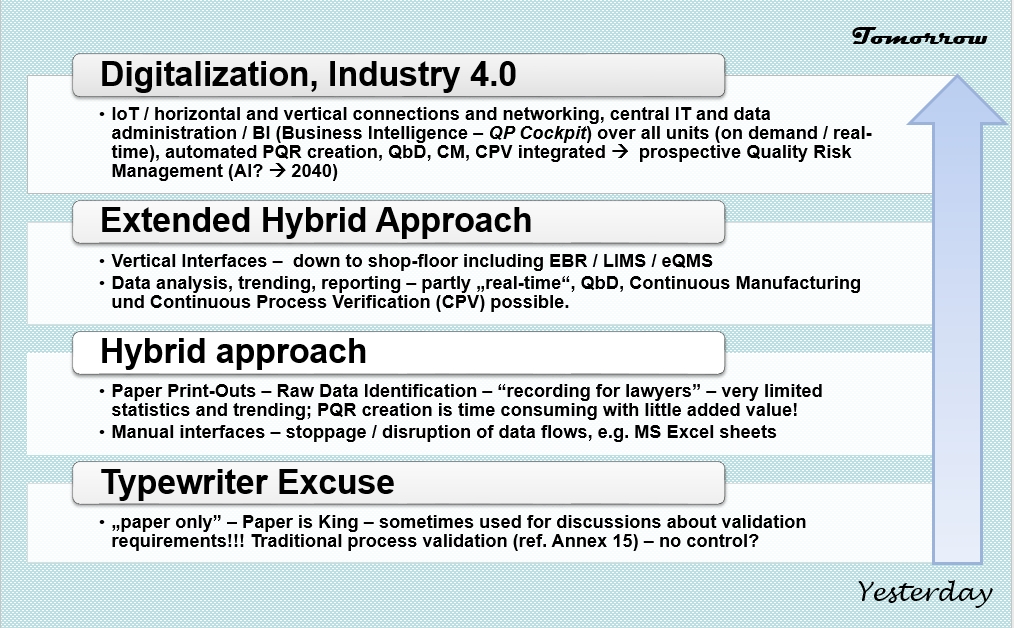
Are You Ready for the Latest Data Integrity Guidance? Part 1: Scope, Data Governance, and Paper Records