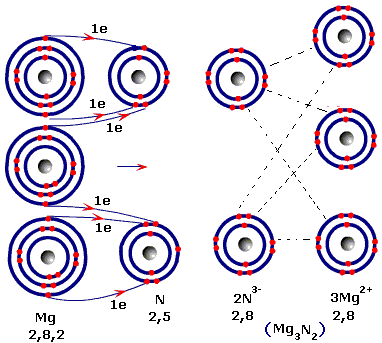
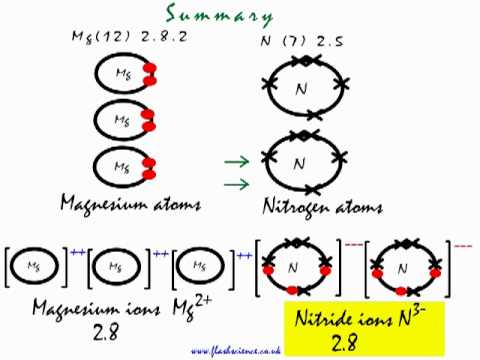
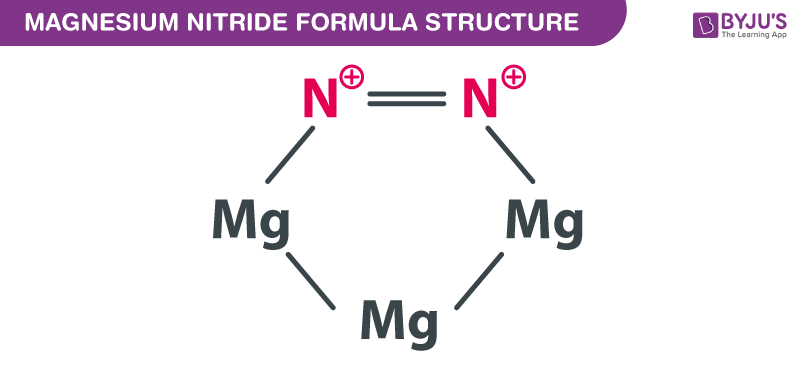
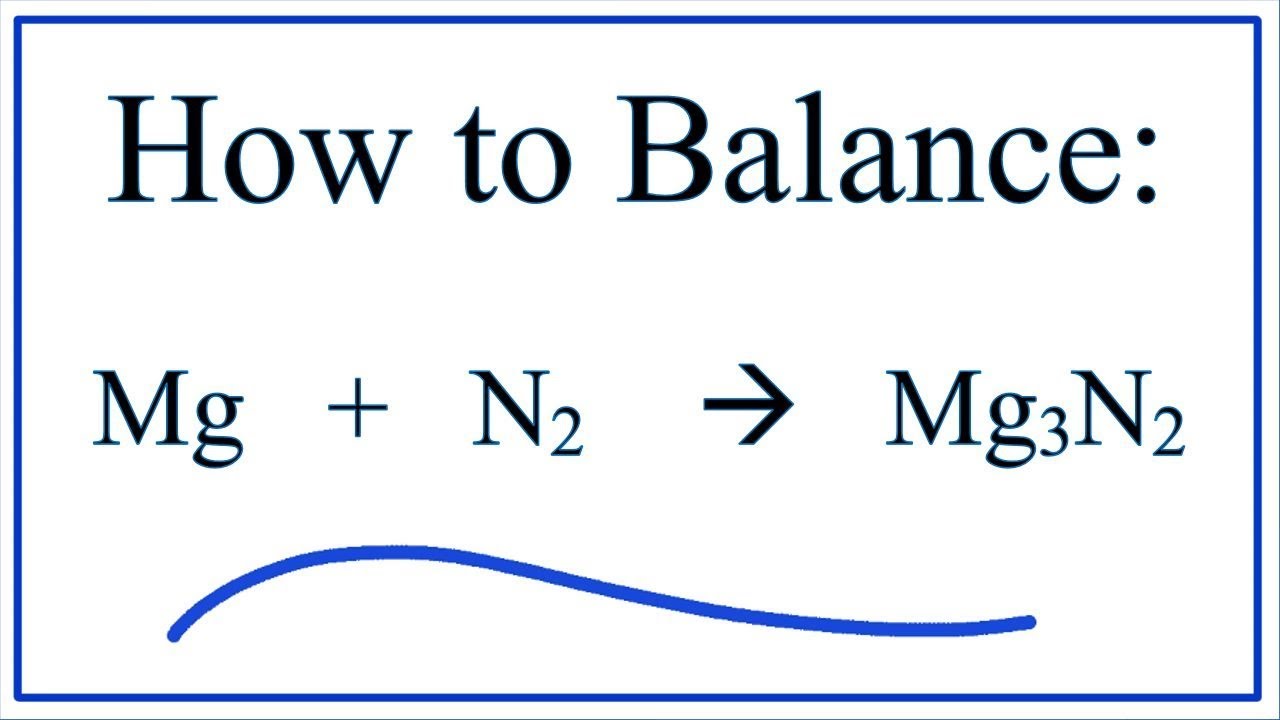
Starting with the atomic electron configurations of Mg and N to forming the corresponding | Homework.Study.com

Carbon monoxide reacts with hydrogen under certain condition to form methanol (CH3OH) . Write a balanced chemical equation for this reaction indicating the physical state of reactants and product, as well as
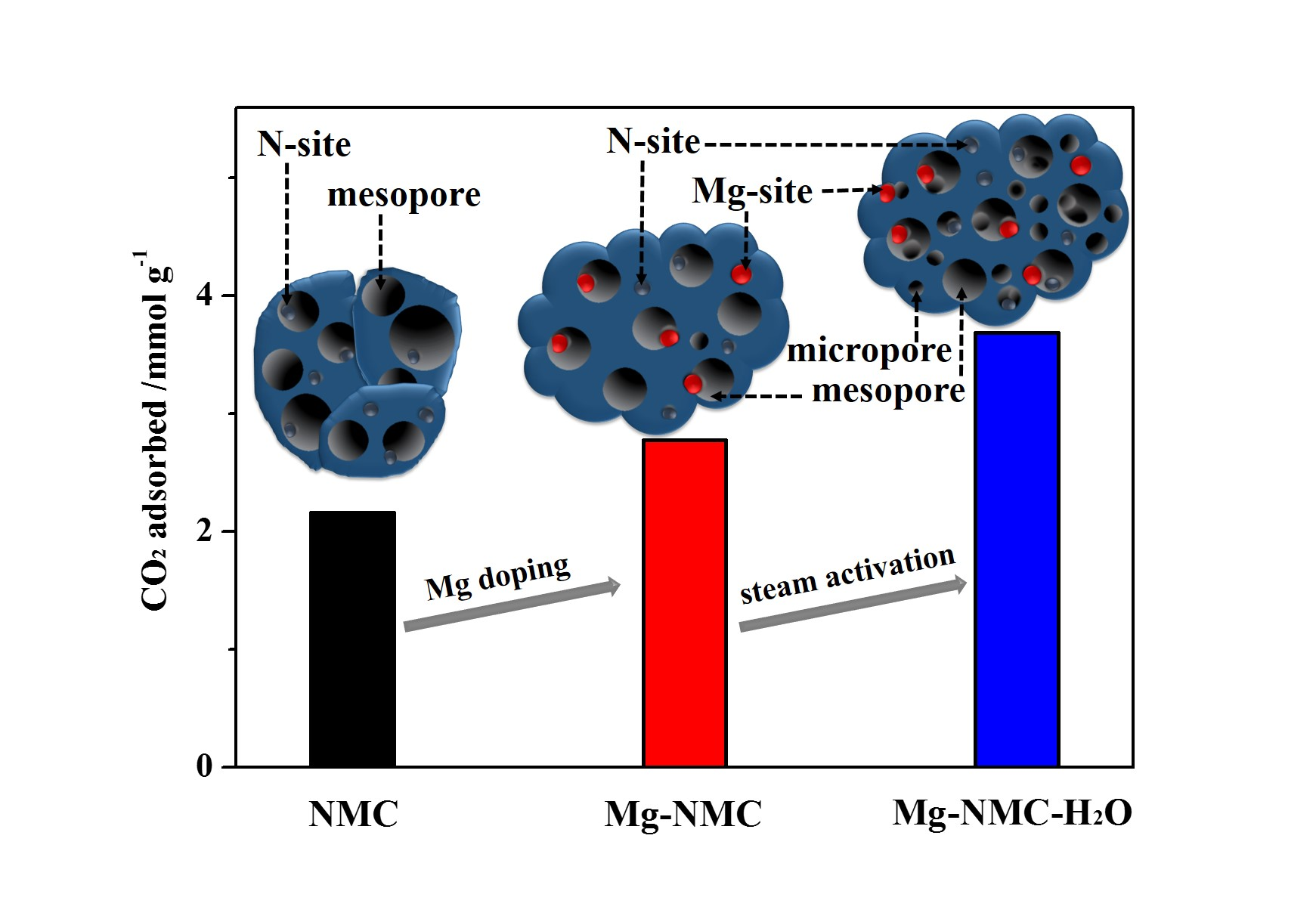
Nanomaterials | Free Full-Text | Magnesium and Nitrogen Co-Doped Mesoporous Carbon with Enhanced Microporosity for CO2 Adsorption
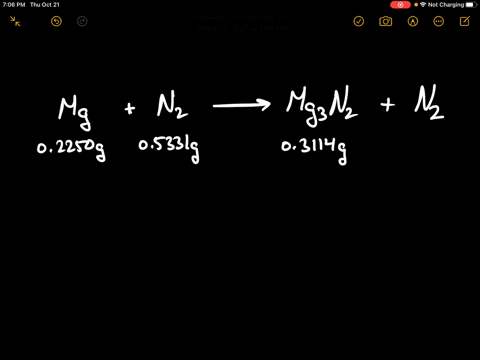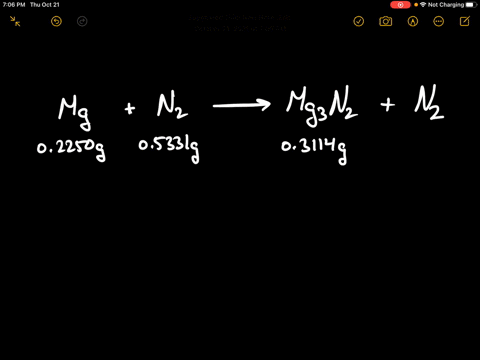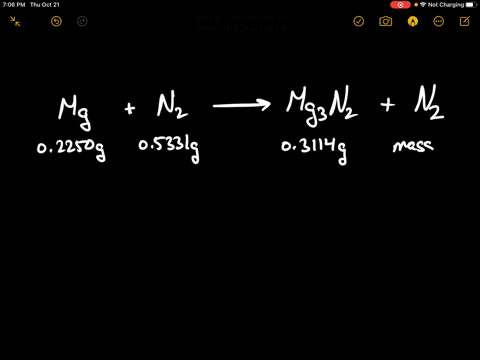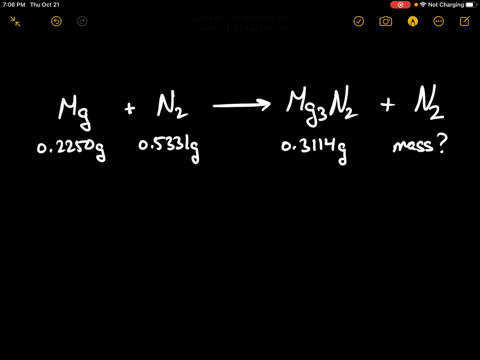
SOLVED:When 0.2250 g of magnesium is heated with 0.5331 g of nitrogen in a closed container, the magnesium is completely converted to 0.3114 g of magnesium nitride. What mass of unreacted nitrogen

SOLVED: The bond between nitrogen atoms in N2 molecules is very strong, making N2 very unreactive. Because of this, magnesium is one of the few metals that react with nitrogen gas directly.

Mineral fertilizer - Magnesium Plus - Omex Agriculture - with trace elements / N / rich in magnesium