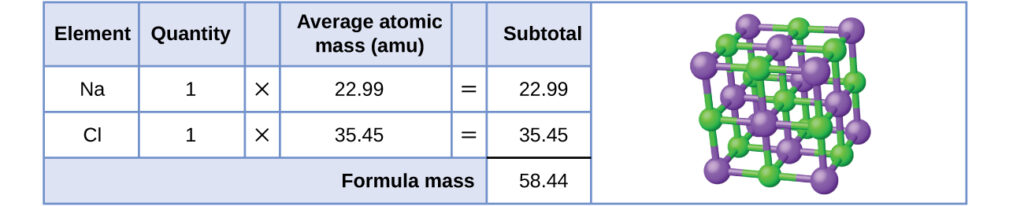
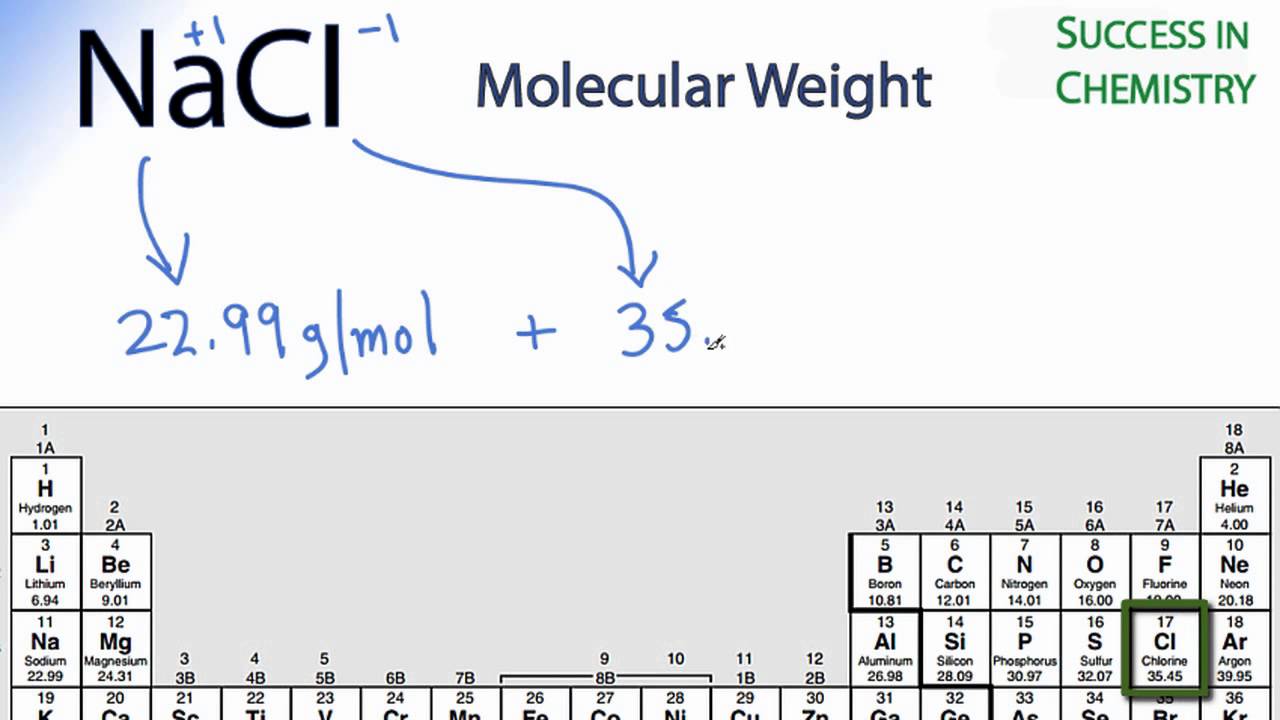
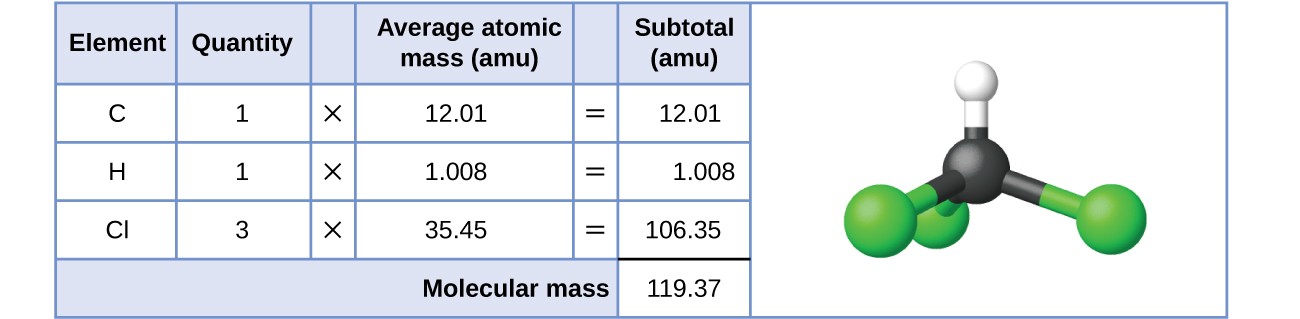
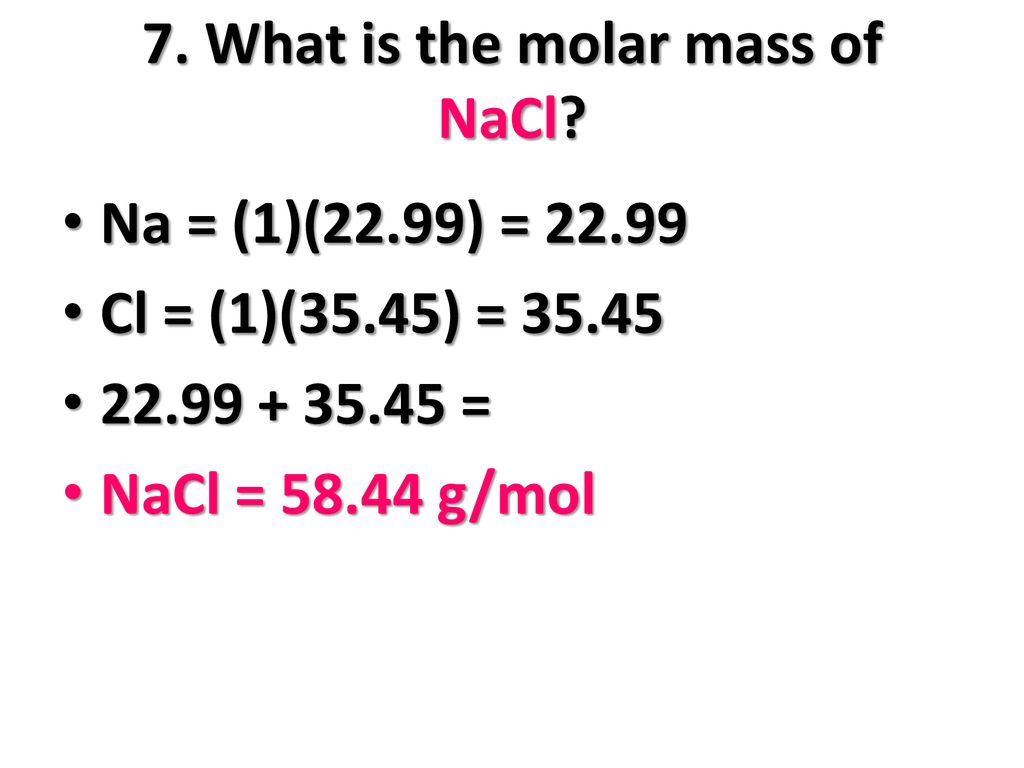Formula mass of NaCl is 58.45 g mol ^-1 and density of its pure form is 2.167 g cm ^-3 . The average distance between adjacent sodium and chloride ions in the

Table I-B Molar Mass of Some Common CompoundsAtomic Mass of each element in a given compound (g) - Brainly.ph
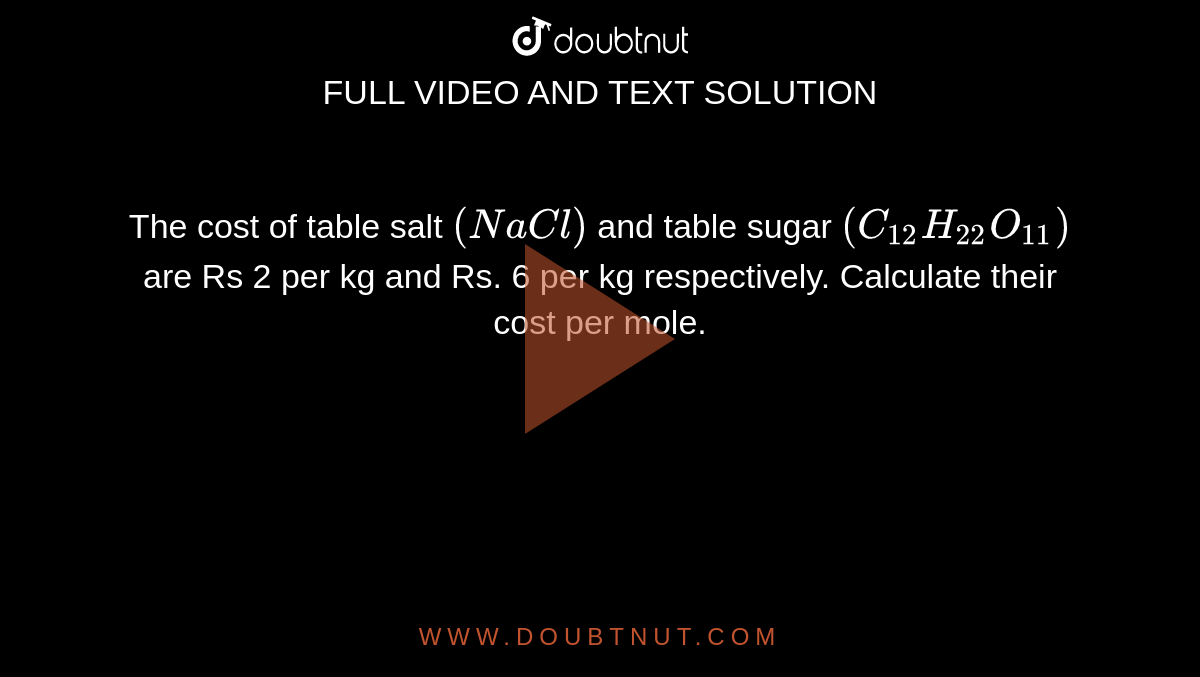
The cost of table salt ( NaCl ) is Rs. 10 per Kg. calculate its cost per mole. ( Molar mass of NaCl is 58.5" gmol"^(-1) )

The reaction, 2A(g) + B(g) 3C(g) + D(g) , is begun with concentration of A and B both at initial value of 1 M . When equilibrium is reached, the concentration of

MOLAR MASS Molar mass of a substance = mass in grams of one mole of the substance. A compound's molar mass is NUMERICALLY equal to its formula mass. Formula. - ppt download

The molar mass of table salt (NaCI) is 58.5 g/mol. What mass of salt is equivalent to 3.00 moles - Brainly.com
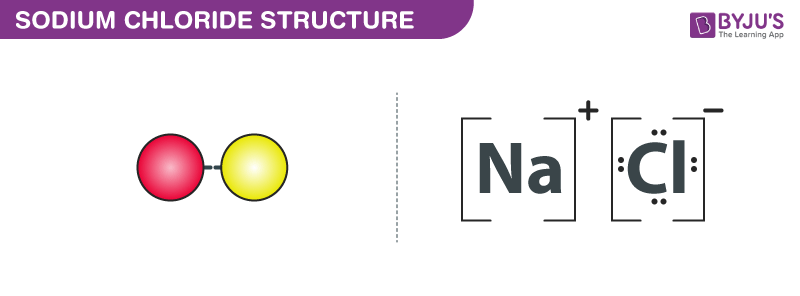
:max_bytes(150000):strip_icc()/sodium-chloride-structure-artwork-160936423-589330f15f9b5874eea7ba04.jpg)